Breakthrough in Phage Research Offers New Antibacterial Strategies
A team of German researchers has unveiled a breakthrough method using antisense oligomers to precisely disrupt phage infection, opening new avenues for targeted antibacterial therapies amid rising antibiotic resistance.
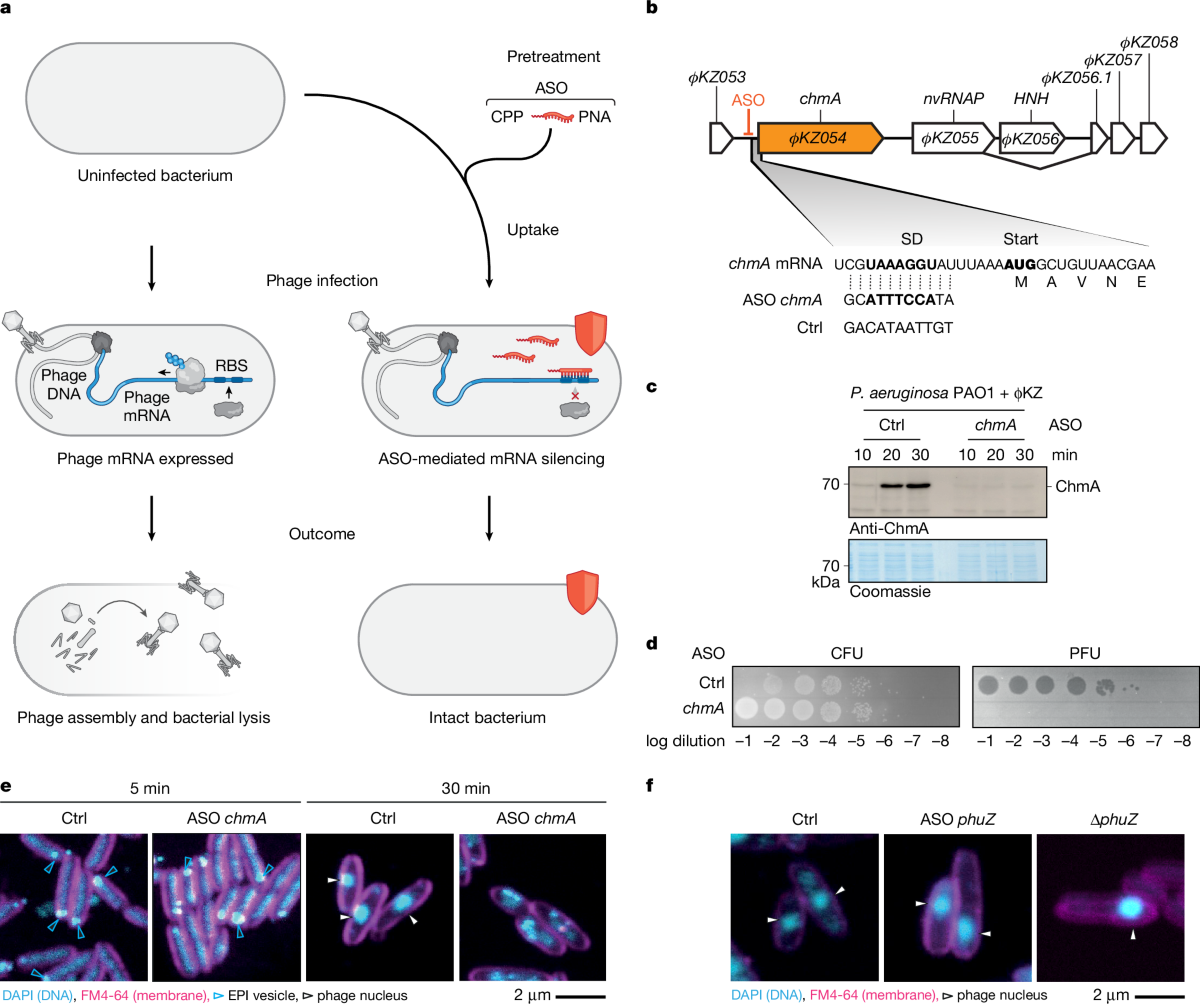
A major advance in phage research was announced on September 10, 2025, as scientists at the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg, Germany, revealed a novel technique to manipulate bacteriophage infection at the molecular level. The study, published in Nature, demonstrates how antisense oligomers (ASOs)—short, synthetic strands of nucleic acids—can be programmed to bind specific phage mRNAs, effectively silencing essential viral genes and halting the phage life cycle.
The research addresses a longstanding challenge in the field: the molecular 'shield' that phages use to protect their genetic material from both bacterial defenses and scientific investigation. By targeting this shield with ASOs, the team was able to systematically knock down individual phage genes, providing unprecedented insight into the genetic choreography of infection and viral replication. This approach was tested on the so-called 'jumbo' phage ϕKZ, which infects the pathogen Pseudomonas aeruginosa and is notable for its large genome and complex infection mechanisms.
Programmable Antibiotics and Antimicrobial Resistance
Unlike traditional antibiotics, which often indiscriminately kill both harmful and beneficial bacteria, phages offer highly specific antibacterial action. However, their therapeutic use has been limited by gaps in understanding how phages interact with their bacterial hosts. The new ASO-based method allows researchers to selectively block the expression of phage genes critical for infection, paving the way for the rational design of 'programmable antibiotics.' According to lead author Jörg Vogel, "Harnessing their therapeutic potential, especially against the backdrop of increasing antibiotic resistance, would be a game changer."
The study's findings are particularly timely as the world faces a growing crisis of antibiotic-resistant infections. By enabling precise control over phage activity, the ASO technique could accelerate the development of phage therapies tailored to target specific bacterial pathogens, potentially restoring the effectiveness of antibacterial treatments where conventional drugs have failed.
Illuminating the Phage-Bacteria Arms Race
The research also sheds light on the intricate molecular battle between phages and bacteria. The team discovered that the phage ϕKZ orchestrates its infection cycle through a sophisticated sequence of gene expression, involving a protective 'phage nucleus' that shields its genome and a switch between different phage-encoded RNA polymerases. By disrupting these processes at defined stages, the scientists were able to map the essential components required for successful infection and identify new targets for therapeutic intervention.
This breakthrough not only advances the fundamental understanding of phage biology but also provides a powerful toolkit for future studies. The ability to perform gene-specific knockdowns in the context of active infection opens new possibilities for exploring viral evolution, host defense mechanisms, and the development of next-generation antibacterial strategies.
Local and Global Impact
The discovery, led by a German research consortium and highlighted by local and international outlets, underscores the global significance of phage research in the fight against antibiotic resistance. As the scientific community continues to seek alternatives to traditional antibiotics, the programmable ASO approach represents a promising step toward safer, more effective antibacterial therapies.